carbon valence electrons|Valence electron : Pilipinas In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence . Tingnan ang higit pa คู่มือการเข้าใช้งานระบบ NEW GFMIS THAI วันที่แผยแพร่ 29/12/2564 ดาวน์โหลด. 4.21.pdf (มีผู้ Download เอกสารแล้ว 183 ครั้ง)
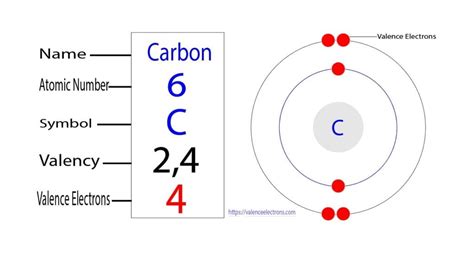
carbon valence electrons,In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence . Tingnan ang higit pa
Electron configurationThe electrons that determine valence – how an atom reacts chemically – are those with the highest Tingnan ang higit pa
The valence shell is the set of orbitals which are energetically accessible for accepting electrons to form chemical bonds.For main . Tingnan ang higit paThe number of valence electrons in an atom governs its bonding behavior. Therefore, elements whose atoms have the same number of valence electrons are often . Tingnan ang higit paValence electrons are also responsible for the bonding in the pure chemical elements, and whether their electrical conductivity is characteristic of metals, semiconductors, or insulators.Metallic elements generally . Tingnan ang higit pa1. Francis, Eden. Valence Electrons. Tingnan ang higit pa Learn the valences of the chemical elements, the number of electrons with .
carbon valence electrons Valence electron Mar 23, 2023
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as . We can see from the electron configuration of a carbon atom—1s 2 2s 2 2p 2 —that it has 4 valence electrons (2s 2 2p 2) and 2 core electrons (1s 2). You will see in .
Learn how elements gain or lose electrons to form ions and ionic compounds. See examples, questions, and explanations about valence electrons and octets.
Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical .Learn how to determine the number of valence electrons for an element using the periodic table. See patterns, examples, and tips for main group and transition metals.

Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its .
carbon valence electronsValence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called . sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . The highest principal quantum number is 2. There are 2 electrons in the 2s subshell and 2 electrons in the 2 p subshell, giving carbon a total of four valence electrons. Bromine’s ground state . The valence electron configuration of carbon atom is 2s 2 2p 2 as shown in the orbital diagram. Figure 1.6g Orbital diagram of valence electrons in carbon atom. Based on the valence bond .Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .
Microsoft Teams. Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. Carbon atoms are always central; b) Connect the central atom with each of the terminal atom by drawing a single bond. 3. For each single bond, subtract two electrons from the total number of valence electrons. 4. Using the remaining valence electrons, complete the octets of the terminal atoms first, and then complete as many as possible .
Bohr diagrams for hydrogen, helium, lithium, carbon, fluorine, neon, sodium, silicon, chlorine, and argon. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration.
Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..Carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in the figure below. Each of these sp 3-hybridized atoms is then bound to four other carbon atoms, which form bonds to four other carbon atoms, and so on. As a result, a perfect diamond can be thought .Total valence electrons pairs in ozone. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. Total electron pairs are determined by dividing the number total valence electrons by two. For, CO 2, Total pairs of electrons are 8. Sketch and selection of center atom of CO 2 molecule. We know carbon has the greatest .

Looking at the 2s 2 2p 2 valence electron configuration of carbon, we might expect carbon to use its two unpaired 2p electrons to form compounds with only two covalent bonds. We know, however, . The standard atomic mass of carbon is 12.0096 and its symbol is ‘C’. The valence electrons are the total number of electrons in the last orbit (shell). The total number of electrons in the last shell .
Valence electron At carbon, with Z = 6 and six electrons, we are faced with a choice. . For chemical purposes, the most important electrons are those in the outermost principal shell, the valence electrons. 2.2: Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts.In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules.Valence is generally understood to be the number of chemical bonds that each atom of a given chemical element typically forms. Double bonds are considered to be . For example, in CO2, carbon needs 6 electrons to fulfill the octet, whereas oxygen needs only 2 electrons. Now, let us quickly go through the steps for creating a lewis structure of any compound. Step 1 – First and foremost we need to calculate the total valence electrons present in the molecule. Care should be taken regarding +, – signs. The four valence electrons of the carbon atom are distributed equally in the hybrid orbitals, and each carbon electron pairs with a hydrogen electron when the C–H bonds form. Figure \(\PageIndex{15}\): The four valence atomic orbitals from an isolated carbon atom all hybridize when the carbon bonds in a molecule like CH 4 with four .
The total valence electrons in the molecule can be calculated by multiplying the valence electrons of each atom. Carbon, which belongs to group 14 of the periodic table, has four valence electrons, while oxygen, belonging to group 16, has six valence electrons. Since CO 2 consists of one carbon atom and two oxygen atoms, the .
4.E: Valence Electrons and Bonding (Exercises) These are homework exercises to accompany Chapter 4 of the Furman University's LibreText for CHE 101 - Chemistry and Global Awareness. Valence electrons are outer shell electrons with an atom and can participate in the formation of chemical bonds. In single covalent bonds, .
carbon valence electrons|Valence electron
PH0 · Valences of the Chemical Elements
PH1 · Valence electrons and ionic compounds (video)
PH2 · Valence electrons (video)
PH3 · Valence electron
PH4 · Valence Electrons Chart for All Elements
PH5 · Valence Electrons
PH6 · Determine valence electrons using the periodic table
PH7 · 3.10: Valence Electrons
PH8 · 2.9: Valence Electrons
PH9 · 10.6: Valence Electrons